Abstract
Background :Proteasome inhibitor-based induction is currently considered standard of care in newly diagnosed multiple myeloma (NDMM) patients (pts) eligible for high-dose therapy and transplant (MEL200-ASCT). The second-generation proteasome inhibitor Carfilzomib proved to be effective, either in combination with Lenalidomide-Dexamethasone (KRd) or with Cyclophosphamide-Dexamethasone (KCd) (Jakubowiak, Blood 2012; Bringhen, Blood 2014).
Aim : The primary objective of this analysis was to compare the efficacy of KRd vs KCd induction in pts eligible for transplantation. The secondary aim was to evaluate the efficacy of KRd vs KCd in different subgroups of pts according to prognostic features, focusing specifically on high-risk pts.
Methods : NDMM pts <65 years were randomized (1:1:1; stratification: International Staging System [ISS] Stage I vs II/III and age ≥ 60 vs < 60 years old) to receive: 4 28-day KCd cycles (carfilzomib: 20/36 mg/m2 IV d 1, 2, 8, 9, 15, 16; cyclophosphamide 300 mg/m2 d 1, 8, 15; dexamethasone: 20 mg d 1, 2, 8, 9, 15, 16) followed by MEL200-ASCT and consolidation with 4 KCd cycles; or 4 28-day KRd cycles (carfilzomib and dexamethasone as above; lenalidomide: 25 mg d 1-21) followed by MEL200-ASCT and 4 KRd cycles; or 12 KRd cycles. After the 4th induction cycle, all patients proceeded with stem cell collection. Post-induction response rates and minimal residual disease (MRD) by multiparameter flow cytometry (8 colors, sensitivity 10-5) were evaluated. In pts who achieved ≥very good partial response (VGPR), sequential centralized MRD evaluation was planned every 6 months starting after induction. For this analysis, data of the two KRd groups were pooled together, since pts had received the same induction. Subgroup analysis of post-induction VGPR rate was performed by using multivariate logistic regression model with interaction terms. Data cut-off was May 31, 2017.
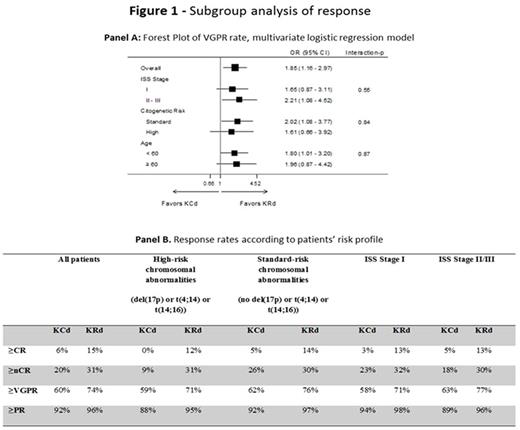
Results : At data cut-off, 309 pts were randomized to KRd vs 154 pts to KCd. Pts characteristics were well balanced: median age was 57 years in both groups, ISS Stage II/III was reported in 48% of pts in KRd and KCd, high-risk chromosomal abnormalities (del(17p) or t(4;14) or t(14;16)) were present in 30% of KRd pts and 34% of KCd pts. After 4 cycles of induction, the rate of ≥partial response (PR) was 96% with KRd vs 92% with KCd; ≥VGPR was 74% with KRd vs 61% with KCd (P=0.01), with 31% vs 20% of pts (P=0.03) achieving ≥near complete response (nCR) and 15% vs 6% (P=0.02) ≥complete response (CR) respectively in KRd and KCd; stable disease was reported in 3% of KRd vs 7% of KCd pts, with only 1% of pts in each group being primary refractory. Subgroup analysis of response rates showed the superiority of KRd vs KCd in terms of ≥VGPR in all the subgroups analyzed (Figure 1 - Panel A). Of interest, the rates of ≥VGPR, ≥nCR and ≥CR were comparable in high- and standard-risk pts treated with KRd (Figure 1 - Panel B). Six-month MRD results were available in a subset of pts (122 KRd and 53 KCd pts): MRD was negative in 66/122 (54%) KRd pts vs 16/53 (30%) KCd pts (P=0.005).
Regarding safety, the rate of ≥1 grade 3-4 hematologic adverse events (AEs) was comparable between KRd and KCd (7% vs 6%), the most frequent being neutropenia (KRd 6% vs KCd 5%). The rate of ≥1 Grade 3-4 non-hematologic AEs was higher with KRd (32% vs 16%, P<0.001), mainly represented by manageable cutaneous toxicity (8% vs 1%, P<0.001) and reversible increase in liver enzymes (8% vs 1%, P<0.001); the rate of grade 3-4 cardiovascular AEs was 5% with KRd and 4% with KCd. Treatment discontinuation for AEs was reported in 4% of KRd and 2% of KCd pts.
Conclusions :KRd induction showed a higher efficacy of KCd in terms of ≥VGPR, ≥nCR and ≥CR rates, with an acceptable safety profile. The superiority of KRd vs KCd was confirmed in high- and standard-risk groups, with response rates in high-risk pts treated with KRd comparable to the ones in standard-risk, suggesting the high efficacy of this regimen even in this subset of pts representing a current unmet medical need. Updated data will be presented at the meeting.
Gay: Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Seattle Genetics: Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees; Mundipharma: Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria; Celgene: Honoraria; Bristol Myers Squibb: Honoraria. Offidani: celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees. Oliva: Takeda: Honoraria; Celgene: Honoraria. Mancuso: Bristol Myers Squibb: Honoraria; Amgen: Honoraria; Janssen: Honoraria; Celgene: Honoraria. Palumbo: Bristol-Myers Squibb: Consultancy, Honoraria, Research Funding, Speakers Bureau; Takeda: Consultancy, Employment, Equity Ownership, Honoraria, Research Funding; Sanofi: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria, Research Funding; Celgene: Consultancy, Honoraria, Research Funding; Genmab A/S: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Research Funding; Janssen-Cilag: Consultancy, Honoraria, Research Funding; Merck: Consultancy, Honoraria, Research Funding; Binding Site: Research Funding. Musto: Janssen: Honoraria; Celgene: Honoraria. Boccadoro: Amgen: Honoraria, Research Funding; Mundipharma: Research Funding; AbbVie: Honoraria; Bristol-Myers Squibb: Honoraria, Research Funding; Sanofi: Honoraria, Research Funding; Janssen: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Celgene: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal